Table 8. Program Outcomes
Overview
New Applications: This table extrapolates the potential effectiveness of the proposed training program based on students who would have been eligible for the proposed training program over the past 5 years, had it existed.
Renewal Applications: This table reports the use of trainee positions (distribution by faculty, year in program, years of support per trainee) and helps evaluators understand the program’s effectiveness over the last 15 years.
Complete Table 8A for programs with pre-docs, Table 8C for programs with post-docs, and both 8A and 8C for combined programs. Table 8B is for Short-Term grant programs, which are unusual submission types at UCLA. If you are submitting for a short-term program contact GSUTraining@mednet.ucla.edu for more specific support.
Overall Instructions
Table 8A (Pre-Docs) & 8C (Post-Docs)
- Part I Those Appointed to the Training Grant (RENEWAL ONLY). List, by year of entry into the program, all trainees who have been supported by this grant at any time during the last 15 grant years. Include those who did not complete the training program for any reason. Update trainee information to reflect current appointments. If the grant has been active for less than 15 years, list all trainees to date. If any trainees were appointed as both pre- & post-docs, they should be reported on Table 8A only.
- Part II Those Clearly Associated with the Training Grant (RENWAL ONLY). List current graduate students at UCLA who are clearly associated, but not currently supported by this training grant; omit column 9 “Subsequent Grant(s)/Role/Year Awarded”.
- Note: “Clearly Associated” refers to trainees with experience similar to those appointed to this grant (i.e. Part I) who are supported by other NIH or HHS awards like fellowships or research grants, but not by this grant. Do not count diversity and administrative supplements as “Clearly Associated,” these trainees are directly associated under the same grant number.
- If you have no “Clearly Associated” trainees, Part II is not applicable.
- Interim Research Performance Progress Report (RPPR)
- In addition to the annual RPPR, an Interim RPPR is required for all renewal applications. If the renewal is not funded, the Interim RPPR will serve as the Final RPPR for the project; if the renewal is funded, the Interim RPPR will serve as the annual RPPR for the final year of the previous competitive segment.
- For each renewal application and interim RPPR, provide updated information that reflects new entrants and updated information for clearly associated students since the last reporting period until 15 years of data have been completed. Do not include data older than 15 years.
- Note: GSU does not provide RPPR support services.
- Part III Recent Graduates (NEW PROGRAMS and PRE-DOC/POST-DOC ONLY PROGRAMS EXPANDING TO INCLUDE BOTH PRE- & POST-DOCS). List all pre-docs who graduated in a field (Table 8A) or post-docs who completed a training experience in a field (Table 8C) from a program similar to the proposed program in the last five years. List by year of entry into the program who would have been eligible for the proposed program if an NIH or other HHS training or related award were available*. Only include columns 4 “Summary of Support During Training” and 9 “Subsequent Grant(s)/ Role/Year Awarded” if they are applicable.
- Part IV Program Statistics (RENWAL PRE-DOC PROGRAMS ONLY). Report on the following:
- Percentage of Trainees Entering Graduate School 10 Years Ago Who Completed the PhD. Trainees must have received support from the training grant at some point during graduate school and completed their PhD or equivalent doctoral degree.
- Average Time to PhD for Trainees in the Last 10 Years. For all trainees appointed to this training grant, calculate the average to one decimal (e.g. 5.5 years).
- Programs that have not received support for at least 10 years (or 2 cycles) should omit Part IV.
- Note on Calculating Data for Part IV:
- Trainees leaving graduate school to transfer to medical school or other doctoral-level professional programs should be counted as part of the “Entering” pool, not as having earned a Ph.D.-equivalent degree.
- When calculating these fields, individuals transferring to or from Ph.D. programs in similar fields at other institutions should be excluded from both “Entering” and “Graduating” cohorts. Time to PhD should be calculated as the period from enrollment in a doctoral program at UCLA to the conferral of a Ph.D. (incl. dual-degree programs). If a trainee earns a master’s degree from UCLA prior to and in conjunction with fulfilling the requirements for the research doctoral degree, or an additional doctoral degree as part of a dual-degree program (e.g., M.D./Ph.D., D.D.S./Ph.D.), time to degree should be calculated from entry into the first degree program.
- Page Limit: None. For more information visit our FAQs.
- Note on Calculating Data for Part IV:
*Eligibility: In most cases, eligible candidates are citizens or non-citizen nationals of the U.S. or permanent residents. They are required to pursue their research training full time and are normally assigned 12-month appointments, but no less than 9 months. Check the specific FOA for full details.
- Pre-docs: Trainees must be enrolled in a program leading to a PhD or equivalent research doctoral degree program. Health-professional students who wish to interrupt their studies for a year or more to engage in full-time research training before completing their formal training programs are also eligible.
- Post-docs: Trainees must have received a PhD, MD, DDS, or comparable doctoral degree at the time of their training grant appointment start date. Check the specific FOA for a list of comparable doctoral degrees. Individuals in postgraduate clinical training who wish to interrupt their studies for a year or more to engage in full-time research training before completing their formal training programs are also eligible.
Summarize data from Table 8 Parts I-III in the Research Training Program Plan's narrative Qualifications of Trainee Candidates and Admission and Completion Records and Progress Report subsections. More time should be spent analyzing this data for renewal applications in order to stress the program’s efficacy.
Helpful Hints
Find Similar Programs
- Example: The PIs proposing a new neuro-urology training program know that a neurosurgery and urologic oncology training program already exist on campus. From running an NIH RePORTER search, they also learn of a neuro-epidemiology grant that has the potential to share eligible trainees so the PIs asked their colleagues to share information about their students that would have been eligible for their proposed program, had it existed. They added these trainees to Table 8A/C.
Guidance by Column
Table 8A: Pre-Docs (Parts I, II, & III)
- Trainee. Last Name, First Name and Middle Initial
- Faculty Member. List up to 2 faculty mentors in the format Last Name, First Name and Middle (list “TBD” if not yet selected)
- Start Date. List date of entry into the trainee’s current degree-granting program in the format MM/YYYY (may precede the appointment to the training grant)
- Summary of Support During Training. Provide the primary source and type of support during each twelve-month period of training in the format TY1, TY2, etc.; do not include individual mentored career development awards (K awards)
- Renewals Only: List the training program being reported in the current renewal in bold
- Doctoral programs: TY1 is when the trainee entered doctoral training, and the final Training Year will be when the degree was granted (for dual-degree programs that do not award both degrees simultaneously, the final Training Year will be when the last degree was granted)
- NIH and other HHS support: List the abbreviation for the awarding institute code and activity code (e.g., NC R01)
- Other sources: Use the categories below to report only the primary source and type of support for each twelve-month period of training
- Sources of Support:
- NSF
- Other Federal (Other Fed)
- University (Univ)
- Foundation (Fdn)
- Non-US
- Other
- None: Leave of Absence (LOA)
- Types of Support:
- Research Assistantship (RA)
- Teaching Assistantship (TA)
- Fellowship (F)
- Training Grant (TG)
- Scholarship (S)
- Other
- Sources of Support:
- Terminal Degree(s) Received and Year(s). Those who left the graduate program without a degree list “none”
- Renewals Only: For trainees currently in the program, list “in training”
- Topic of Research Project. A brief description or title
- Initial Position. Complete for trainees who have completed or left the program; if individuals hold joint appointments/positions, only list the primary; if information is not available list “unknown;” for each position, the table should include the following fields in this section:
- Position title
- Department/division
- Institution
- Workforce Sector:
- Academia
- Government
- For-profit
- Nonprofit
- Other
- Principal Activity:
- Primarily research
- Primarily teaching
- Primarily clinical
- Research-related
- Further training
- Unrelated to research
- Current Position. Utilize the same fields as Initial Position (column 7); for trainees who completed or left the graduate program, leave this section blank
- Subsequent Grant(s)/Role/Year Awarded. If applicable, list up to 5 fellowship, career development, or research grant support awards obtained from any source after the individual completed training. For NIH and other HHS support, list the abbreviation for the awarding institute, activity code, role, and year awarded (e.g., NC R01/Staff Scientist/2011); applicable for any role held
Guidance by Column
Table 8C: Post-Docs (Part I, II, & III)
- Trainee. Last Name, First Name and Middle Initial
- Doctoral Degree(s) and Year(s). Trainee’s degree(s) and the year(s) awarded
- Faculty Member. List up to 2 faculty mentors in the format Last Name, First Name and Middle (list “TBD” if not yet selected)
- Start Date. List date of entry into the trainee's postdoctoral research program in the format MM/YYYY (may precede the appointment to the training grant); entry year is the first year of post-doc research experience excluding non-research clinical training
- Summary of Support During Training. Provide the primary source and type of support during each twelve-month period of training in the format TY1, TY2, etc.; do not include individual mentored career development awards
- Renewals Only: List the training program being reported in the current renewal in bold
- Doctoral programs: TY1 is when the trainee entered doctoral training, and the final Training Year will be when the degree was granted (for dual-degree programs that do not award both degrees simultaneously, the final Training Year will be when the last degree was granted)
- NIH and other HHS support: List the abbreviation for the awarding institute code and activity code (e.g., NC R01)
- Other sources: Use the categories below and report only the primary source and type of support for each twelve-month period of training
- Sources of Support:
- NSF
- Other Federal (Other Fed)
- University (Univ)
- Foundation (Fdn)
- Non-US
- Other
- None: Leave of Absence (LOA)
- Types of Support:
- Research Grant (RG)
- Fellowship (F)
- Training Grant (TG)
- Other
- Sources of Support:
- Degree(s) Resulting from Postdoctoral Training and Year(s). If program does not offer degrees list “none”
- Renewals Only: For trainees currently in the program, list “in training”
- Topic of Research Project. A brief description or title
- Initial Position. Complete for trainees who have completed or left the program; if individuals hold joint appointments/positions, only list the primary; if information is not available list “unknown;” for each position, the table should include the following fields in this section:
- Position title
- Department/division
- Institution
- Workforce Sector:
- Academia
- Government
- For-profit
- Nonprofit
- Other
- Principal Activity:
- Primarily research
- Primarily teaching
- Primarily clinical
- Research-related
- Further training
- Unrelated to research
- Current Position. Utilize the same fields as Initial Position (column 8); leave this section blank for trainees who completed or left the graduate program
- Subsequent Grant(s)/Role/Year Awarded. If applicable, list up to 5 fellowship, career development, or research grant support awards obtained from any source after the individual completed training. For NIH and other HHS support, list the abbreviation for the awarding institute, activity code, role, and year awarded (e.g., NC R01/Staff Scientist/2011); applicable only for PD/PI or other senior role (i.e., co-investigator, faculty collaborator, or staff scientist)
Sample Table 8
Note: Follow NIH formatting guidelines – this is a visual demonstration and does not reflect NIH formatting or accurate information/data.
Table 8A. Program Outcomes: Predoctoral
Part I. Those Appointed to the Training Grant
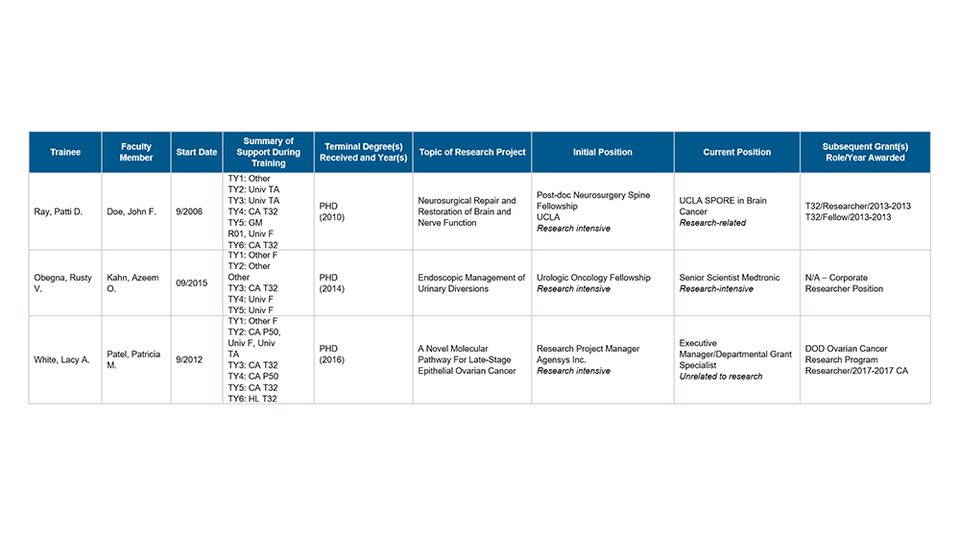
Part II. Those Clearly Associated with the Training Grant
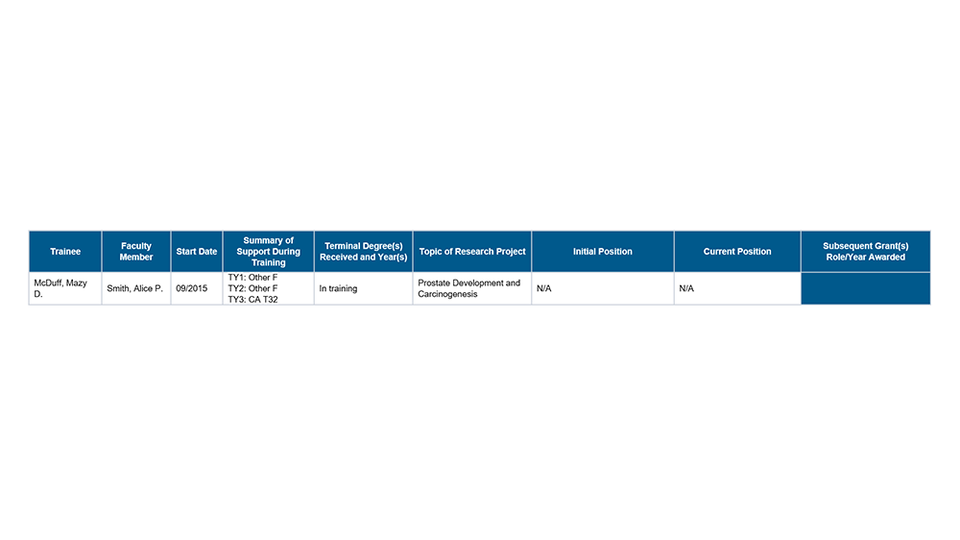
Part III. Recent Graduates
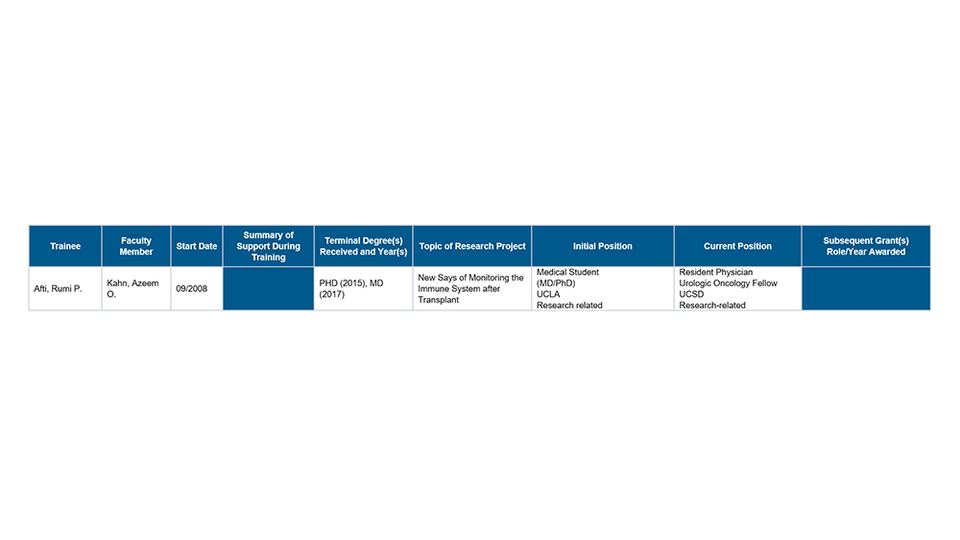
Part IV. Program Statistics
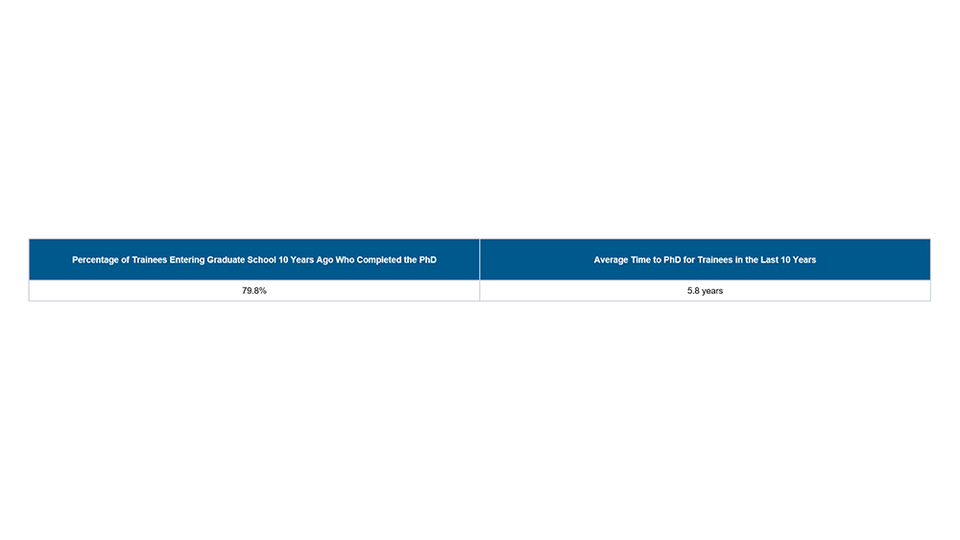
Table 8C. Program Outcomes: Postdoctoral
Part I. Those Appointed to the Training Grant
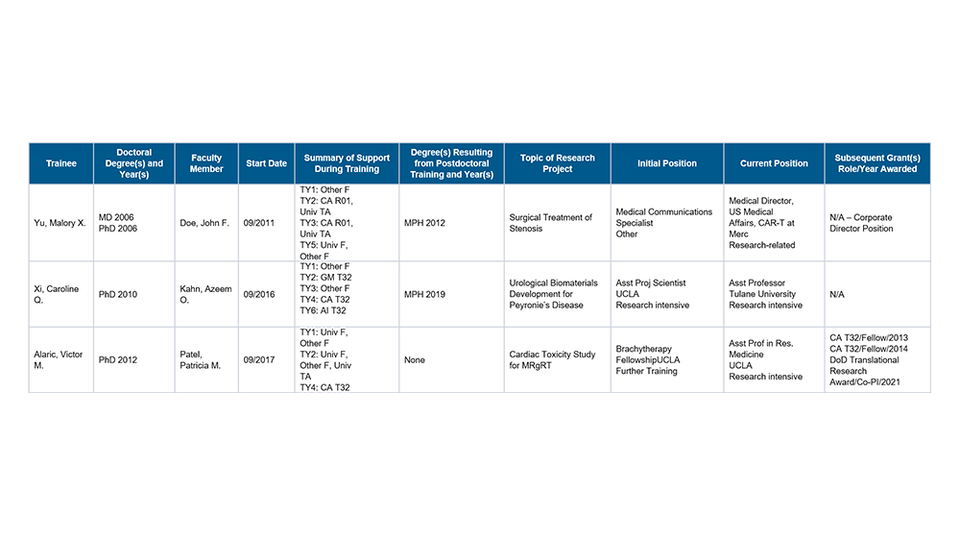
Part II. Those Clearly Associated with the Training Grant
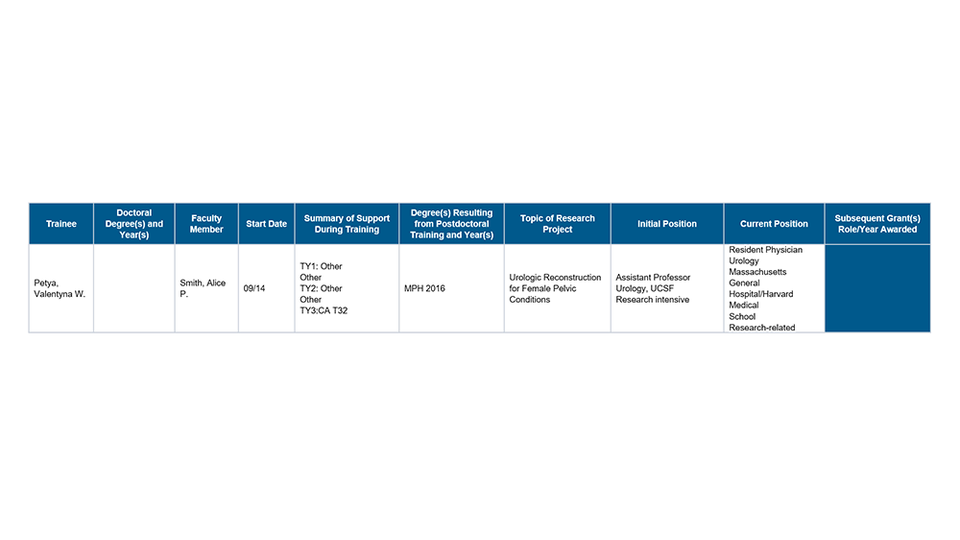
Part III. Recent Graduates
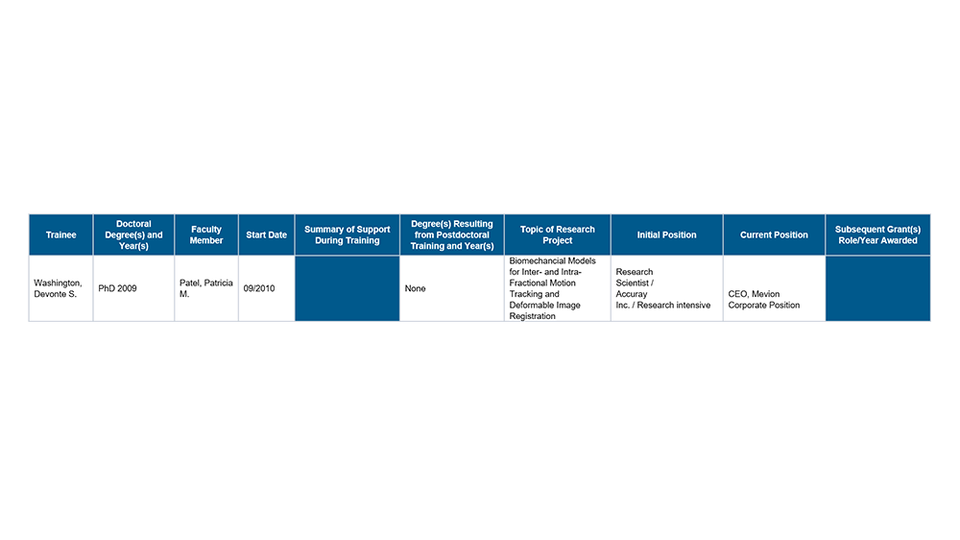
Templates
- NIH Blank Template: Table 8A (Microsoft Word)
- NIH Blank Template: Table 8B (Microsoft Word)
- NIH Blank Template: Table 8C (Microsoft Word)
- Contact GSUTraining@mednet.ucla.edu for formatted Microsoft Excel templates
NIH Resources
-
Data Table Form Library (Please note as of January 2023 NIH is still utilizing FORMS G for Data Tables)
- Consolidated Sample Data Tables and Instructions (Microsoft Word)
- Blank Data Table Template (Microsoft Word)
- Data Table FAQs